

May 2014
IN THIS ISSUE:
![]()
- NIH opens hospital to outside scientists, tackles disease on many fronts
- Local students learn about careers beyond the classroom
- Take your child to work day offers hands on learning
- Refusing 'failure': A patient's hope for the future of women's health and fertility
- Leadership series expands conversation on authority
- Third class of research scholars chosen for yearlong program
- Why do teens participate in medical research?
- NIH women's symposium focuses on future
- Easier access to medical tools now available
- Clinical Center remembers former drug researcher
- Exercise, save money and help the environment: Bike to Work May 16
- Upcoming Events
Print this Issue ![]() (333 KB)
(333 KB)
ABOUT CC NEWS:
![]()
Published monthly by the Office of Communications and Media Relations. News, article ideas, calendar events, letters, and photographs are welcome. Submissions may be edited.
Clinical Center News
National Institutes of Health
Building 10, 10 Center Drive
Room 6C-420,
Bethesda, MD 20892-1504
Tel: 301-594-5789
Fax: 301-402-4984
Molly.hooven@nih.gov
QUICK LINKS:
![]()
Bench-to-Bedside (B2B) Proposal Request
Letters of intent to apply for a B2B award are being accepted through June 18, 2014 for the two-year awards designed to seed new projects that propose to translate basic science findings to clinical applications or vice versa. Projects can be exclusively among intramural investigators or can include collaborations between intramural and extramural investigators. Proposals can fall into the following categories: AIDS; behavioral and social sciences; dietary supplements; minority health; rare diseases; women's health; and, general (intramural-only) projects. Questions? Email BedsidetoBench@mail.nih.gov or view additional details on the submission cycle online.
NIH opens hospital to outside scientists, tackles disease on many fronts
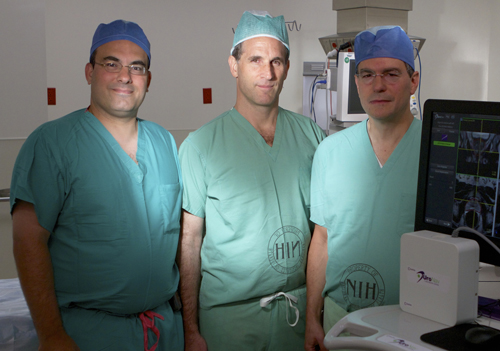 |
Dr. Peter Pinto (NCI), Dr. Brad Wood (CC) and Dr. Peter Choyk (NCI), along with colleagues at NIH and the University of Michigan Medical School, will be working on integrative molecular imaging and sequencing of prostate cancer as part of one of the new grants awarded. |
With NIH's awarding of 10 new projects this spring, non-government researchers are now embarking on a new kind of collaboration with government scientists at the Clinical Center. This opportunity involves three-year, renewable awards of up to $500,000 per year, along with access to the research hospitals extraordinary facilities to scientists from academia and industry across the country.
"We are very excited about opening the doors of the Clinical Center to our extramural colleagues who will bring additional cutting-edge research projects and new partnerships that will enrich ongoing efforts translating scientific discovery into tomorrow's cures and in partnering institutions around the country," said Dr. John I. Gallin, director of the Clinical Center.
The awards will support projects on a variety of diseases and health conditions that affect children and adults in the U.S. and worldwide such as Nieman Pick C, childhood leukemia, prostate cancer and malaria.
Although intramural scientists often collaborate with scientists outside the NIH campus, the new program is expansive and engages a large number of institutes and centers in a coordinated funding opportunity. Extramural grantees from academia and industry will have direct access to the wide-ranging, cutting-edge resources of the Clinical Center.
Outside scientists will be able to test promising laboratory discoveries using emerging technologies and tools and collaborate on clinical protocols, often for extraordinarily rare diseases, in partnership with NIH investigators to help advance disease diagnosis, treatment and prevention.
View the full list of research partnerships and awards.
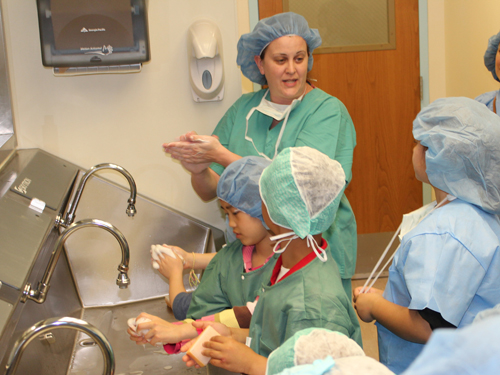
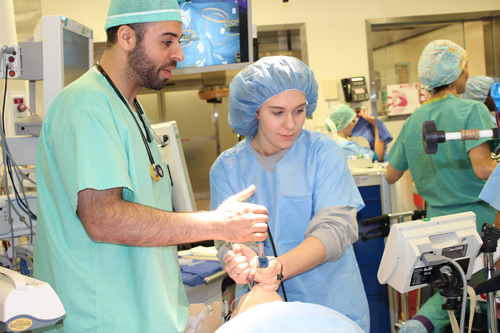
Take your child to work day offers hands on learning
| ||||||||
|
| |||||||
| ||||||||
Refusing 'failure'
A patient's hope for the future of women's health and fertility
In 2008, Goldnar Miamee went to her OB/GYN and said, "I know this may sound funny, but I feel like a menopausal woman." With a laugh, her doctor said "Oh, you're too young for that."
She thought nothing of it at the time. But a few years later, after trying to get pregnant without success, she was diagnosed with primary ovarian failure.
"I was not ready to accept that as a diagnosis, so I found out about the NIH study," Miamee said. "Coming here was a life changing experience. I finally was able to accept my condition which, is actually primary ovarian insufficiency."
Primary ovarian insufficiency (POI) is a condition in which women younger than 40 have ovaries that stop releasing eggs, or release them only intermittently, and stop producing normal amounts of the hormones estrogen, progesterone and testosterone, or produce them only intermittently.
Miamee is a part of the Eunice Kennedy Shriver National Institute of Child Health and Human Development natural history study Ovarian Follicle Function in Women with Spontaneous Primary Ovarian Insufficiency. The study aims to learn more and improve treatments for young women with POI and clarify the differences from menopause.
During the study, Miamee and many participants came to realize that POI is not just about fertility.
"Not only did I lose my chance of having children, but I also was living with hot flashes and mood swings, depression and possible bone loss," she said. As part of the study, she was prescribed hormone replacement therapy to increase their levels. "I didn't take them for the first six months because I thought I don't need it. I finally decided to take the hormone replacement therapy and found them to help tremendously. POI was really affecting my life before; but then was under control."
While recruitment ended in 2011, the natural history study continues to take new scientific approaches to learning more about POI. Miamee, along with 500 women ranging from age 18 to mid-30s, now have the opportunity to have genetic testing done on the samples of blood they provided.
Dr. Lawrence Nelson, principal investigator of study, and his team are working with researchers at Washington University in St. Louis through a collaborative agreement on conducting whole exome sequencing on the women's samples.
"If we can find genetic causes of this then we can eventually develop tests that are predictive of who's going to get it," Nelson said. "Women could have the genetic testing done and determine if they are likely to develop POI and either change their plans to get pregnant earlier, or adopt or [say] my career's really important to me so now I know let's focus on that."
But as the NIH team was preparing the protocol, they came across an ethical dilemma.
"We're doing research; we're looking at POI; this is the main reason for doing it, but what happens if we find a gene that's related to breast cancer in our research? What do we do about that?" Nelson said.
Miamee, who was consulted in the writing process of the new consent form for the genetic testing, said, "I would definitely want to know, but I do see that there are women who didn't want to know. To me, that's one of the positive sides of this whole study."
After extensive conversations with leading genetic experts, they decided that the academic researchers would notify NIH, who would then consult with patients, if they come across one of 57 genes specific to a disease or condition other than POI, such as familial hypercholesterolemia or malignant hyperthermia susceptibility. A genetic counselor and support team will be made available to those with an abnormal gene that has been clinically validated, which is expected to occur in only one in 100 women. There are also known 'next steps' for patients to follow for each of the 57 genes, so they can take action to stay healthy.
"Even if [NIH] never finds the result, I'm OK with the fact that people are spending the time and initiative to even try," Miamee said. "There were other diseases where people participated in a study and maybe in their lifetime they never got answers, but at some point, I'm confident they will, maybe past my time, but I'm sure something good will come out of it."
The long-term goal is to transfer a fully-integrated patient care and research program to the community where it would be self-sustaining by people supportive of women with POI. Miamee has already taken the first step to reach out to the community by creating a group called Positive Optimistic and Involved for women with POI. "I just wanted to use my good experience and knowledge to help," she said.
Leadership series expands conversation on authority
 |
Laura Lee presents on 'Speaking Truth to Power.' |
On April 9, the Clinical Center Office of Workforce Management and Development held the third session of the 2014 Clinical Center Leadership Development Brown Bag Series. Laura Lee, special assistant to the deputy director for clinical care at the Clinical Center, spoke to an overflowing Medical Board Room of nearly 50 people on examining the impact of authority gradients on communication and patient safety and the key role communication plays in our work day.
"We use words to educate patients, counsel employees, order tests and hand off critical information. It's more than just talk, its communicating," Lee said. "[Its] an incredibly important part of the quality care we provide our patients."
The last two sessions are Mindfulness in the Workplace May 12, presented by Dr. Rezvan Ameli, clinical psychologist with the National Institute of Mental Health and Using Self-Awareness to Appreciate & Manage Differences June 4, presented by Denise Ford, director of Clinical Center patient and guest relations.
Sessions are held in the Medical Board Room (4-2551) at noon. Employees can learn more online (NIH Staff Only) or call 301- 594-9548 for presentation materials.
Third class of research scholars chosen for yearlong program
In late March, 43 students were selected for the Medical Research Scholars Program (MRSP) 2014- 2015 class to engage in a mentored basic, clinical or translational research project on the NIH campus that matches their interests and career goals. The new class represents 34 institutions, 39 medical students, three dental students and one veterinary student. The scholars will start arriving in July to begin a yearlong experience in a range of biomedical research from bench to bedside and beyond.
"I cannot begin to express how excited I am to have been accepted," said Cortlyn Brown, Yale University School of Medicine. Future scholar Raju Chelluri from State University of New York Upstate Medical Center agreed, "It's truly an honor. I'm excited for [the] year!"
A total of 85 applicants were invited for an interview and to enjoy dinner and presentations by NIH leaders and current and former scholars on Sunday March 2. Plans for the applicants to come to campus Monday March 3 for interviews were halted by ice and snow-covered streets in the early morning hours. Despite the winter snowstorm, the Office of Clinical Research Training and Medical Education staff and NIH investigators worked diligently to provide all applicants with a meaningful and quality interview experience by phone.
"Thank you for all your hard work [that] weekend," said Taufiq Salahuddin, a student from Wake Forest University School of Medicine who's been chosen to participate in the upcoming class. "I was impressed by the manner things were handled. What I saw was a very close-knit group of current scholars and a program that cares about its students."
Ninety students have participated in the program since it began in 2012.
Why do teens participate in medical research?
Teens feel they are contributing to society when they participate in medical research even if there are no direct benefits to them, according to a study led by Clinical Center staff.
Chief of the Bioethics Department, Christine Grady, and staff member Dave Wendler led the study, Parental Permission and Adolescent Assent and Decision Making in Clinical Research, from 2008 to 2010 in collaboration with Lori Wiener and Sima Zadeh of the National Cancer Institute pediatric oncology branch, and Drs. Ben Wilfond and Doug Diekema and staff at the Seattle Children's Hospital.
In analyzing the data, they've come to some interesting conclusions.
"There are many teens participating in medical research," Grady said. A lot of changes occur during adolescence which means diseases and possible treatments might affect teens differently than other age groups. "We need teens to participate in research to understand how to provide better medical care for them."
Federal regulations governing the protection of human subjects in medical research require adolescents provide assent and parents or legal guardians provide permission for most research. The federal regulations don't provide specific guidelines for what the assent or permission process should look like, Grady said.
"Enrolling in a medical research trial is a significant decision. We want to gain a better understanding of what teens understood about the research and how much they were involved in the decision-making," Grady said. "To make the experience as ethical as possible and to protect them, we need to understand it from their perspective."
Grady and team conducted structured interviews with 147 teens, ages 13-17, and Seattle Children's Hospital staff interviewed 30 teens. All had been enrolled in a clinical study in the previous six months.
Teens answered about 100 questions and a parent or guardian was also interviewed about the teen's experience during enrollment.
"This was a remarkably well-balanced cohort," Grady said. Teens were 51 percent female, 49 percent male with about 20 percent at each age. About 20 percent were healthy volunteers, and the rest ranged from moderately ill to severely ill.
"We could not have interviewed this many teens without the help of the Clinical Center's doctors, nurses and other staff who referred so many patients to us," Grady said.
Survey questions ranged from overall satisfaction with the enrollment process to specific elements of how the decision was made to volunteer.
A high percentage of teens felt supported throughout the decision-making process, and normally the teen and parent made the decision to enroll together. Despite emerging independence, in this situation, teens generally want parents' input and advice.
Recommendations from the findings of this study include: provide teens with detailed information about what to expect at each step of the research and tailor the assent process to them where possible. Grady said they have also recommended teens be given the option of signing an assent form as teens reported this gives them a sense of power and control in the process.
The survey addressed the ethics of involving teens in medical research that did not benefit them. "What we learned from the surveyed teens was they did not feel exploited by such studies," Grady said. "They understood they were making a valuable contribution to help other people, and they were willing to do that."
The researchers found teens and parents overwhelmingly reported they would be willing for the teen to complete a non-invasive procedure such as an extra blood draw or chest x-ray and more than half would also be willing to do a skin biopsy even if it was not going to benefit them but was for the research.
Parents said the offer of money would make no difference in their decision about these procedures. Healthy teens were more likely than teens with serious illnesses to report money would make them more likely to agree to extra tests.
About 25 percent reported they felt pressure to enroll from parents and friends and in some cases from medical staff and researchers too.
"We went into it thinking that younger teens and those with more severe illnesses would have notable differences from older teens and healthy volunteers in feeling pressure to participate in research or satisfaction with the process. This was not the case," Grady said.
Articles on the study have been published in Pediatrics Official Journal of the American Academy of Pediatrics [disclaimer] and the Journal of Adolescent Health [disclaimer].
NIH women's symposium focuses on future
|
Easier access to medical tools now available
The Clinical Center hosted a conference and hands-on training session ![]() (427 KB) April 3 to introduce NIH-funded tools: the Patient-Reported Outcomes Measurement Information System, the NIH Toolbox for Assessment of Neurological and Behavioral Function and the Quality of Life in Neurological Disorders. NIH invested in the assessment tools to create a platform for clinical research and patient care.
(427 KB) April 3 to introduce NIH-funded tools: the Patient-Reported Outcomes Measurement Information System, the NIH Toolbox for Assessment of Neurological and Behavioral Function and the Quality of Life in Neurological Disorders. NIH invested in the assessment tools to create a platform for clinical research and patient care.
Single-sign-on versions of the tools have been created for secure use behind the NIH firewall. Investigators will now be able to easily access the tools for patient care, clinical research and clinical interventions/trials.
Leslie Wehrlen, Clinical Center nurse specialist research, was one of 12 speakers including institute/center directors and the developers of the tools. She's worked extensively with Margaret Bevans, Clinical Center clinical nurse scientist, who's one of only two NIH researchers to pilot-test the tools. Wehrlen talked about translating patient-centered outcomes into clinical research. "Regardless of what measure you choose, you can really customize this approach to meet your needs of your population," she said.
The tools provide a valid approach to studying patient symptoms and other outcomes, often even allowing for apples-to-apples comparisons. They help minimize patient burden when asked to answer questions about their disease and how a treatment may be helping with quality of life. They can also be utilized to design treatment plans and manage chronic diseases.
Clinical Center remembers former drug researcher
 |
Photo of Dr. Albert Sjoerdsma from the cover of Modern Medicine in 1966. |
Dr. Albert Sjoerdsma, a leading Clinical Center pharmacologist and clinical investigator, died Feb. 27 at his home in Southern Shores, N.C.
Sjoerdsma was a major figure in the field of clinical pharmacology, the study of how drugs affect people, and was among the pioneers of mechanism-based drug discovery, targeting enzymes in critical biological pathways.
He came to NIH in 1953 as a senior investigator in the National Heart Institute (now known as the National Heart, Lung and Blood Institute) experimental therapeutics branch and was named chief of the branch in 1958. He trained many young physicians who went on to have distinguished careers in clinical pharmacology. His lab focused on the biochemical and clinical aspects of vasoactive amines, especially serotonin and norepinephrine. The anti-hypertensive action of alpha-methyldopa was discovered in his lab in 1958 and became a top-10 prescription drug in the 1960s, remaining on the market to this day.
"Al was both a mentor and a friend; he was full of life, energy and ideas. He gave you support and the freedom to find your own way," said Dr. J. Richard Crout, a former clinical associate in Sjoerdsma's branch and later Director of the Food and Drug Administration's then-Bureau of Drugs.
In 1970, Dr. Sjoerdsma sat on a selection committee which awarded a Merck, Sharp and Dohme International Fellowship to Dr. Juan Lertora who was an aspiring young scientist at Tulane University School of Medicine at the time and now current director of the clinical pharmacology program at the Clinical Center.
"That [fellowship] gave me the boost to pursue my training in clinical pharmacology," Lertora said. "This man was a giant. He was a pioneer in the field; he had a long and productive life."
After he left NIH in 1971, Sjoerdsma went to Richardson-Merrell to head its international research center in France. He rose to become president of Merrell-Dow Research Institute. Under his watch, the institute developed a drug that has saved thousands of lives from an African sleeping sickness, a parasitic disease spread by tsetse flies that, left untreated, is uniformly fatal.
A memorial service will be held in Kitty Hawk, N.C., in September.
Exercise, save money and help the environment: Bike to Work May 16
Bike to Work Day 2014, will be celebrated at NIH and across the nation on Friday, May 16. Last year, more than 150 Clinical Center employees registered for Bike to Work Day. Rain or shine and even in the snow he cycles to his office and studio at the Clinical Center. "You just need to make very wide turns when it's snowing and wear waterproof pants if it's pouring outside," Branson said with a laugh. The following NIH pit stops will be open to cyclists on Bike to Work Day: in front of Bldg.1, Rockledge Drive at the Rock Springs Business Park and Fallsgrove Village Center in Rockville. Pre-registration is necessary to receive a t-shirt at the Bldg. 1 pit stop. Don't forget to indicate NIH as your employer and Bldg. 1 as the preferred choice of pit stop when you register online. For the first time event participants, Branson recommends finding back roads to avoid car traffic on major roads. Burn calories instead of gas and don't forget to smile when you see him photographing the event! |
Upcoming Events
Lectures will be streamed and archived
NIH Medical Research Scholars Program
Scientific presentations
May 12-13, 2014; 8:30 a.m. - 5:30 p.m.
Lipsett Amphitheater
Fourty five students will present their medical research.
Contemporary Clinical Medicine: Great Teachers Lecture
Antiplatelet therapy: from serendipity, to random screening, to rational molecular design
May 14, 2014; noon - 1 p.m.
Lipsett Amphitheater
Presented by Barry S. Coller, MD, Rockefeller University Hospital.
Office of Research on Women's Health Scientific Forum
Sex differences in neuroscience: past, present, and future perspectives
May 14, 2014; 2 p.m. - 4 p.m.
Lipsett Amphitheater
Presented by Story Landis, PhD, NINDS, Vicky Holets Whittemore, PhD, NINDS, Cheryl Bushnell, MD, Wake Forest Baptist Stroke Center, Margaret McCarthy, PhD, University of Maryland School of Medicine.
NIH Director's Wednesday Afternoon Lecture Series
Antiviral defense mechanisms at the mucosal surfaces
May 14, 2014; 3 p.m. - 4 p.m.
Masur Auditorium
Presented by Akiko Iwasaki, PhD, Yale School of Medicine.
Clinical Center Grand Rounds Lecture
Pediatric bipolar disorder, irritability, and new approaches to psychiatric diagnosis
When does bipolar disorder begin? Genetic approaches to age at onset
May 21, 2014; noon - 1 p.m.
Lipsett Amphitheater
Presented by Ellen Leibenluft, MD, NIMH and David T. Chen, MD, NIMH.
NIH Director's Wednesday Afternoon Lecture Series
Interplay between genes and environment in insulin resistance and metabolic syndrome; the unique role of the gut microbiome
May 21, 2014; 3 p.m. - 4 p.m.
Masur Auditorium
Presented by Ron Kahn, MD, Harvard Medical School
Clinical Center Grand Rounds Lecture
Ethical challenges of academic medicinal chemistry: synthetic marijuana as dual use research
May 28, 2014; noon - 1 p.m.
Lipsett Amphitheater
Presented by Harvey V. Fineberg, MD, PhD, Harvard School of Public Health and Aidan Hampson, PhD, NIDA.
NIH Director's Wednesday Afternoon Lecture Series
ABC transporters: structures, functions, and reaction mechanisms
May 28, 2014; 3 p.m. - 4 p.m.
Masur Auditorium
Presented by Kaspar Locher, PhD, ETH Zürich.
NOTE: PDF documents require the free Adobe Reader.
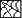 The information on this page is archived and provided for reference purposes only.
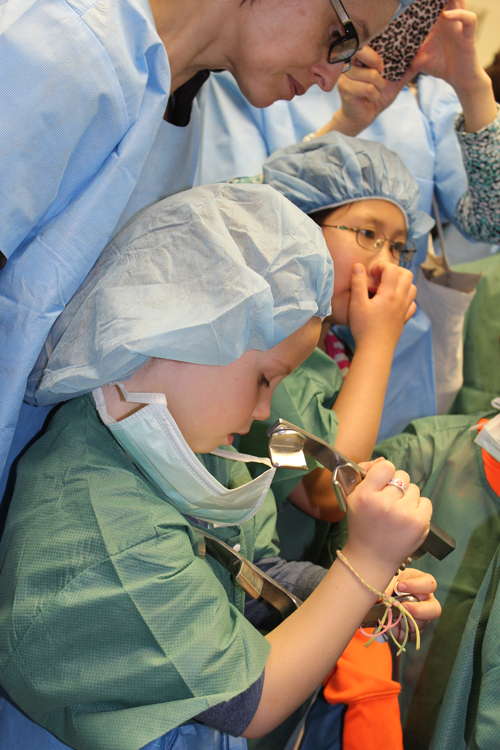
The information on this page is archived and provided for reference purposes only.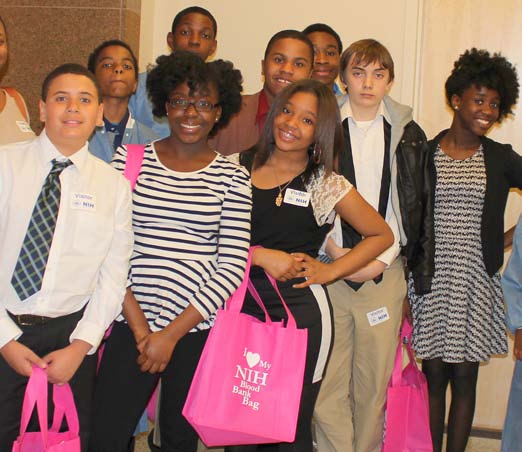 Over 30 students from Benjamin Banneker Middle School in Burtonsville, including those pictured above, toured the Clinical Center April 8 as part of the hospital's ongoing effort to inspire young people from diverse communities to pursue careers in health and science.
Over 30 students from Benjamin Banneker Middle School in Burtonsville, including those pictured above, toured the Clinical Center April 8 as part of the hospital's ongoing effort to inspire young people from diverse communities to pursue careers in health and science.

 Terra Miller (left), program analyst in the Clinical Center
Terra Miller (left), program analyst in the Clinical Center 