Laboratory Services Section (LSS) Research Program
Willy A. Flegel, MD
Chief, Laboratory Services Section
Immunogenetics and molecular immunohematology are the translational research topics of the Laboratory Services Section (LSS) in the Department of Transfusion Medicine (DTM). Molecular methods were introduced first in HLA laboratories, and red cell genotyping followed approximately by the year 2000. Clinical applications have been expanded and refined ever since. We promote moving transfusion medicine laboratories rapidly into the molecular arena, because molecular tools have been shown to improve patient care to a level that cannot be achieved by any available serology alone.
Milestones that we accomplished include: the elucidation of the physical structure of the RH gene locus, which led to the understanding of the molecular basis and mechanism of the RHD deletion (see Figure 1); the determination of RHCE as the ancestral RH gene, while the RHD is the duplicated gene; the molecular bases of the weak D phenotypes and the prevalent DEL phenotype; the definition of ERMAP as the gene encoding the Scianna blood group antigens; and the application of red cell genotyping in hematopoietic progenitor cell transplantation.
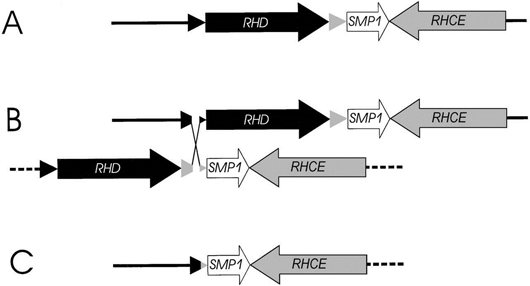
Figure 1. Molecular basis of Rh negative. (A) The physical structure of RHD and RHCE genes on the short arm of chromosome 1. (B) An unequal crossing-over event between the 2 Rhesus boxes (triangles) was triggered by their nucleotide sequence homology. (C) Resolving the crossed-over chromosome yields the RH gene structure of the extant RHD negative haplotype that is prevalent in humans. Adapted from Blood 95(2000)3667.
Our current research interests include: red cell genotyping; relationship of genotype, phenotype and function of blood group proteins; stem cell surface antigens in vivo and during ex vivo expansion; and immunogenetics in hematopoietic stem cell transplantation focusing on HLA and KIR genes. We develop molecular immunohematology and immunogenetics methods for blood transfusion, hematopoietic progenitor cell transplantation and immune monitoring, and apply these methods to clinical services for patient care. The projects represent translational research in support of NIH Clinical Center patients, often in collaboration with other NIH investigators.
Our translational research has led to several methods with clinical applications. Their implementation, for example by commercially available kits and platforms, has increased blood component safety. The availability varies among health-care systems, notably between North America and Europe. The advent of such Conformité Européenne (CE)-labeled test kits has rendered it technically and legally possible, within the specifications of the CE-certification process for in vitro diagnostic devices in the European Union, to replace several blood group serology tasks by red cell genotyping; this is not the case in the US at this time.
Strengthening the clinical approach by using molecular technologies that were previously restricted to applied research fits well into DTM’s goals of providing cutting edge clinical services. DTM laboratory services are offered nationwide as a strong reference laboratory resource for selected immunohematology and HLA work. We will demonstrate the applicability of the available technologies in the population, improve available technologies, facilitate implementation in national blood group laboratories and establish a dataset to gauge the cost-benefit. We envision molecular typing as the future for resolving several remaining issues in blood component compatibility. Molecular typing benefits many patient groups, such as pregnant women and patients with sickle cell disease, who are vulnerable to immunization or subsequently become difficult to supply with compatible blood.
LSS is a research environment combining access to unique patient and blood donor specimens with an established expertise in molecular technology and a primary interest in translational research.
Blood Services Section (BSS) Research Program
Kamille West, MD
Chief, Blood Services Section
The Blood Services Section is responsible for maintaining a volunteer donor registry of whole blood and apheresis donors sufficient to meet 100% of the transfusion needs of Clinical Center patients; for maintaining a research donor registry sufficient to meet 100% of the needs of NIH investigators for blood components for in vitro research use; for providing therapeutic apheresis services and blood stem cell collections to support clinical transplant and other patient-care protocols; and for maintenance of one of the largest hospital-based National Marrow Donor Program registries in the country. In line with these functions, the section conducts investigator-initiated and collaborative research focused in four areas:
- Establishing programs to study and treat iron deficiency and other eligibility determinants in volunteer blood donors, with the goal of maximizing blood donor eligibility status.
- Developing a comprehensive program for management of patients with hereditary hemochromatosis in the Blood Center, involving clinical and molecular studies of response to phlebotomy therapy.
- Optimizing the safety and efficacy of apheresis donations.
- Collaborating with NIH investigators to perform prospective trials of therapeutic apheresis and evaluate novel hematopoietic transplantation strategies.
Cell Processing Section (CPS) Research Program
David Stroncek, MD
Chief, Cell Processing Section
New Assays for Assessing Cellular Therapies
The production of cellular therapies is complex. Most clinical cell therapy products require cell mobilization, collection, subset isolation, in vitro or in vivo stimulation, and culture over a period of several days. The production of some cellular therapies involves serial isolation steps and multiple stimulation and/or culturing steps. Cellular therapy product manufacture is further complicated by donor or patient genetic and physiological heterogeneity. The final product is often markedly different from the starting material.
Despite these complexities cellular therapies must be of high quality. Cellular therapies must provide the desired clinical affect without resulting in adverse effects. An adequate dose of cells must be provided to each patient, each product must meet release specifications, and lot-to-lot variation should be minimized.
In order to produce consistently high quality products, quality assurance has become a critical part of cellular therapy laboratories. Cell therapies must be safe, pure, sterile, stable, and potent. To ensure that cell therapies have these properties, they are tested in many ways both during and at the end of their production. The analysis of cell therapies has become an important part of the delivery of effective clinical cellular therapies.
The goal of these studies is to develop novel methods to assess the quality of cellular therapies. Traditional analytic assays including ELISA, flow cytometry, and ELISPOT have many limitations. Better assays are needed to assess the complex and multiple functions of cellular therapy products, some of which are not well understood. Gene expression profiling using microarray technology has been widely and effectively used to assess changes of cells in response to stimuli and to classify cancers. Preliminary studies have shown that the expression of noncoding micro RNA which play an important role in cellular development, differentiation, metabolism and signal transduction can distinguish different types of stem cells and leukocytes. Both gene and micro RNA expression profiling have the potential to be important tools for assessing cellular therapies. In addition, these assays could be used to identify biomarkers that correlate with the safety, purity, and potency of cellular therapies; validate that therapies prepared using different methods are equivalent; and assess changes in the cellular therapy manufacturing process. The development of these assays will lead to safer and more effective therapies for all patients being treated with cellular therapies.
Objectives
- Develop novel molecular methods to access cellular therapies
- Identify and validate biomarkers for cell potency, purity, and viability
- Validate biomarker expression by the cellular therapies with their clinical effectiveness
Development of New Cellular Therapies
The cell therapy laboratory invests a considerable amount of resources in the development of new cellular therapies. While the development of most of these cellular therapies are initiated in the laboratory of clinical investigators, the Cell Processing Laboratory must scale up and validate these procedures. For many protocols this process involves considerable scientific, medical, regulatory and laboratory input from the Cell Processing Section.
Infectious Diseases Section (IDS)
The Infectious Diseases Section conducts a large-scale, investigator-initiated, and collaborative research program. The long-term goals of this research program are to: 1) study and prevent transfusion-associated infections; in the past, these studies focused on transfusion-associated hepatitis, but have now expanded to investigate all potential transfusion-transmitted agents; 2) explore new technologies for the detection and study of blood-transmitted agents including gene array and deep sequencing technology; 3) characterize novel and established blood-transmitted agents, their infectivity, and their susceptibility to inactivation/neutralization; 4) prospectively study donors infected with hepatitis C virus (HCV) to determine the natural history and long-term outcome of this infection; 5) study the immunologic, virologic and gene-associated differences between HCV chronic carriers and those who spontaneously recover; 6) study mechanisms of HCV binding to B lymphocytes and downstream effects using deep sequencing and functional assays; 7) study the mechanisms of HCV binding to erythrocytes and relationship to HCV-related immune complex diseases; 8) study epidemiology and pathogenesis of transfusion-transmitted arboviruses, including Chikungunya virus, dengue virus and Zika virus; investigate the potential for infection enhancement and/or increased severity of disease with co-infection
