
| Home | Contact Us | Site Map | Search |
 |
||||
|
|
|
|
|
| |
|
|
This file is provided for reference purposes only. It was current when it was produced, but it is no longer maintained and may now be out of date. Persons with disabilities having difficulty accessing information may contact us for assistance. For reliable, current information on this and other health topics, we recommend consulting the NIH Clinical Center at http://www.cc.nih.gov/.
|
|
|
|
Clinical Center News, Building 10, Room
1C255, National Institutes of Health, Bethesda, Maryland 20892. (301)
496-2563. Fax: 402-2984. Published monthly for CC employees by the
Office of Clinical Center Communications, Colleen Henrichsen, chief. News,
articles ideas, calendar events, letters, and photographs are welcome.
Deadline for submission is the second Monday of each month. |
HHS Secretary Shalala tapped physicians, scientists, and health-care managers from some of the nation's top hospitals and from across the NIH to serve on the newly appointed board.
John J. Finan, Jr., president and chief executive officer of the Franciscan Missionaries of Our Lady Health System in Baton Rouge, will chair the 17-member group.
"The Clinical Center at NIH is the country's premier medical research facility," said Secretary Shalala. "The board's experience and expertise will enhance the hospital's ability to support research that stands to enhance the lives and health of each American."
"The National Institutes of Health depends on the Clinical Center to
provide an efficient, responsive environment for its conduct of clinical
research," added Dr. Harold Varmus, NIH director.
"Secretary Shalala has assembled an outstanding group of professionals
to add to the hospital's ability to carry out that mission."
Changing how the 43-year-old, Clinical Center is governed topped the list of improvements for the hospital suggested by a team of reviewers appointed by Secretary Shalala in 1995.
She named Dr. Helen Smits, former deputy administrator of the Health Care Financing Administration, to chair the team that scrutinized how the Clinical Center carries out its business. Dr. Smits serves on the new board.
A major recommendation in the team's report, released in January, included a blueprint for the newly named Board of Governors to draw on the expertise of leaders from outside organizations and from inside NIH.
"The board's role is to bring added value to the great work already being done at NIH and at the Clinical Center," explained Finan, who was a member of that original evaluation team. "The board members have a depth and breadth of experience that will strengthen the systems and processes of the hospital and, ultimately, the ability to support scientific research."
Named to the board from outside the NIH are: Dr. J. Claude Bennett, president of the University of Alabama at Birmingham; William B. Kerr, chief executive officer of the Medical Center at the University of California-San Francisco; Dr. Stephen C. Schimpff, executive vice president of the University of Maryland Medical Center, Baltimore; Dr. Helen L. Smits, president and medical director of HealthRight, Inc., Meriden, Conn.; and Ellen M. Zane, network president of Partners in HealthCare System, Inc., Boston.
Finan, Kerr and Dr. Schimpff had served as external consultants to the Options Team.
Appointed to the board from NIH are: Dr. Patricia A. Grady, NINR director; Dr. Jeffrey M. Hoeg, chief of the cell biology section of the Molecular Diseases Branch, NHLBI; Dr. Carl Kupfer, NEI director; Dr. Griffin P. Rodgers, chief of the molecular hematology section, Laboratory of Chemical Biology, and Dr. Allen M. Spiegel, scientific director, NIDDK; Dr. Susan Swedo, acting scientific director, NIMH; and Dr. Robert Wittes, director of NCI's Division of Cancer Treatment, Diagnosis, and Centers.
Drs. Grady, Hoeg, and Rodgers had been members of the Options Team.
Four positions on the board remain to be filled.
"We are privileged to have such a distinguished group of advisors to help us chart the future course of change necessary for the Clinical Center to continue its tradition as a center of excellence in clinical research," said Dr. John I. Gallin, CC director.
He and Dr. Lynnette K. Nieman, chairman of the Medical Executive Committee, will serve as ex officio members of the board. (by Sara Byars)

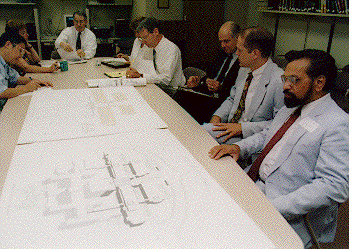
Dr. Harold Varmus (center front), NIH director, welcomed the new CC Board of Govenors during their meeting here Oct. 21. Board members are (front from left) William B. Kerr, University of California-San Francisco; Dr. Jeffrey M. Hoeg, NHLBI; Dr. Helen Smits, HealthRight, Inc.; John J. Finan, Jr., board chairman, president and CEO, Franciscan Missionaries of Our Lady Health System; Dr. John Gallin, CC director; Dr. Patricia Grady, NINR; Ellen M. Zane, Partners in HealthCare System, Inc.; and Dr. Griffin P. Rodgers, NIDDK. In back are Dr. Carl Kupfer, NEI; Dr. Allen M. Spiegel, NIDDK; Dr. Stephen C. Schimpff, University of Maryland Medical Center; Dr. J. Claude Bennett, University of Alabama at Birmingham; Dr. Lynnette K. Nieman, Medical Executive Committee; Dr. Susan Swedo, NIMH; and Dr. Robert Wittes, NCI.
 Members tour
Members tourDr. John Gallin, CC director, presented and the board endorsed a budget assessment plan designed to give the Clinical Center a stable, three-year foundation of funding based on how much clinical research each institute typically conducts and the institutes' plans for future programs. The plan addresses a major recommendation from last year's Options Team that the CC has its own budget that is no less stable than the rest of the NIH intramural programs.
Under the plan, 80 percent of each institute's intramural clinical research budget will be earmarked for the hospital's fixed costs, such as nursing staff, pharmacy services, and supplies. The Board of Governors and the NIH director will review this assessment annually. Twenty percent will be applied to variable costs-money needed to pay for special aspects of specific protocols, for example. This portion of the assessment will be adjusted each year, based on the institutes' plans and prior year's use.
Institutes may appeal the assessments directly to the NIH director or the board, an option Dr. Gallin termed "essential."
Having an envelope of funding to work with will help the Clinical Center develop ways to carry out its business more efficiently, Dr. Gallin said. Those savings will go into a common pool and may be reinvested in clinical research initiatives the following year. If an institute cancels a projected program, it has first crack at applying the already-committed money to its other programs. If they don't do that, the money will go into a common pool that the other institutes may compete for.
"The institute directors agreed in October that we would have a three-year trial of this proposal, which began in FY96," Dr. Gallin said.
"The process will make the institutes' assessments more predictable because they will know what they're going to be committed to well in advance. The proposal will be evaluated after the three-year pilot."
Dr. Harold Varmus, NIH director, underscored the importance of fiscal responsibility in the conduct of clinical research in his welcoming remarks to the board.
"Clinical research is in a period of unusual turmoil and excitement,"
he said, "and we are making a variety of efforts to try to be sure
that the NIH, as an institution dedicated to the improvement of health,
is well positioned to take advantage of what science is producing, despite
the challenges that result from the change in the way health care is being
financed. We've reached a time in the study of human biology when the fruits
of molecular biology, genetics, cell biology, and other disciplines have
the potential for making a tremendous impact upon prevention and treatment
of disease. At the same time we have revolutionary changes in the provision
of health care, which is changing the way medicine is practiced [and] the
way it's financed." (by Sara Byars)
Go to Board of Governors NIH News Advisory
 John J. Finan, Jr., (seated left) presided over the Board of Governor's first meeting here last month. With him is Dr. John Gallin, CC director.
John J. Finan, Jr., (seated left) presided over the Board of Governor's first meeting here last month. With him is Dr. John Gallin, CC director.Finan had chaired the committee of external experts that worked hand-in-hand with last year's Options Team assembled by HHS Secretary Shalala.
The group's mission? To find ways to improve efficiency at the CC without compromising the quality of patient care and clinical research.
Before taking his current position last July, Finan was senior executive officer for alternate site services of the BJC Health System in St. Louis. There he was responsible for system-wide program development and operations relating to ambulatory care; senior service, including home health, hospice, and long-term care; occupational medicine; ground and air transportation; community health; and physician services.
He was president and senior executive officer of the health system's Barnes Hospital from 1992-1996. The 1,208-bed hospital serves as the major teaching affiliate for the Washington University School of Medicine. Finan joined Barnes as a vice president in 1984 and later served as chief operating officer.
Finan holds a B.S. in economics from Louisiana State University at New Orleans and an M.B.A. from Loyola University of the South. He is a fellow in the American College of Health Care Executives and serves as preceptor for a number of health-care executive graduate programs, with a faculty appointment at Washington University School of Medicine health administration program.
Before the board convened for their first meeting on Oct. 21, Finan spoke to CCNews about the group's work ahead.
What do you see as the board's primary mission in the long term?
To help the management of the NIH Clinical Center improve the ability to
serve patients through the skills and expertise that members of the board
bring from our various roles both outside and inside the NIH. The board's
role is to bring added value to the great work being done here by strengthening
the systems and processes that support patient care and scientific research.
What is first on the board's agenda?
Understanding the organization, its challenges and its opportunities. The
Clinical Center leadership will set forth a plan we can oversee and add
value to. [CC Director] Dr. Gallin and his team have done a very good job
putting together an orientation package for us.
The Options Team has performed a thorough review of the organization and there are specific objectives and action plans contained in the Options Team report. All of this provides an inventory of information to quickly learn the Clinical Center's organization, mission, the challenges, and the opportunities.
What do you see as the board's biggest challenge?
Any organization's adjustment to a new structure is change. If we manage
the change well, we will be extremely successful. The concept of a Board
of Governors is new to the Clinical Center and to NIH. There's a real enthusiasm
among Clinical Center and NIH staff, and the individual members of the board
take their appointments seriously and want to help the Clinical Center better
serve patients and scientific research conducted here.
What will the board's combination of experience and expertise bring to
the Clinical Center?
Each member of any board brings a unique background of experience, talent,
and perspective. That's certainly true of this board as well. Secretary
Shalala selected us because of our combined experience in health-care operations
and financing. That experience matches well with the challenges and opportunities
identified by the Clinical Center leadership.
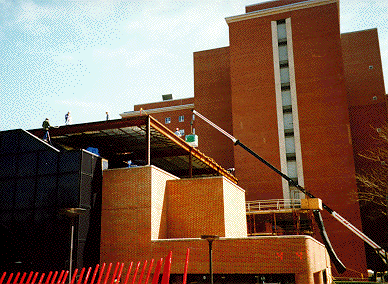
CRC construction carries a $310 million price tag, with funding phases spread
over several years, and is slated to begin in this fiscal year. The current
budget signed into law by President Clinton contains precise language allowing
NIH to contract for the full scope of the new project even though future
year appropriations will be needed to complete funding.
Repairs to the CC garage, expansion of the underground utility tunnel system, construction for a new clinical research facility, and a new fire station will cost almost 1,300 parking spaces over the next several years. Some of those parking spaces will be gone forever.
Here's the math, according to the NIH Transportation Management Plan. There are 9,000 spaces on campus for 15,800 employees. That's about half a parking space for each employee. Well, for each .57 employee, to be precise. And that exceeds the maximum allowable parking ratio for federal installations, which is exactly half a space per person. That puts us over the limit by about 1,100 spaces. The National Capital Planning Commission and the Montgomery County Planning Board have repeatedly asked that NIH reduce its parking "excess" to conform with federally mandated limits. The commitment to reduce excess parking was incorporated into the NIH Master Plan as a condition of approval by the reviewing agencies.
When building projects begin, expect construction workers to snap up the nearby spaces such as those along Cedar Lane.
"When all the construction is under way, if will be extremely difficult to find parking on campus after 7:30 a.m.," predicts Stella Serras-Fiotes, master planner in the DES facilities planning and programming branch. "That's why we're working hard to let employees know about alternate parking and transportation plans."
Here's what is in the works and what the parking toll will be:
Utility tunnel expansion project--Lot 13C will lose 26 spaces
until January 1997. From February to November 1997, Lot 10C will drop 75
parking spaces.
Clinical Center essential maintenance and safety program--
In parking lot 10K, 50 spaces were closed in September. Expect them to be
gone for up to three years. An additional 20-30 spaces may be removed in
early 1997 for other building 10 projects.
Clinical Center parking garage restoration--The building 10
garage will lose up to 400 spaces during various stages of the project.
Demolition in the garage is slated to begin this month. As part of the project,
strike 25 spaces in temporary lot T5 on Convent Drive.
Consolidated laboratory facility--Lot 13C loses 315 spaces
permanently starting in May 1997.
Clinical Research Center (CRC)--The facility will be built
to the north of the existing building 10. Site projects, which must be completed
before actual construction of the CRC, will include a temporary drop off
and entrance at the south of the hospital. This will replace the current
entrance to the ACRF after the relocation of Center Drive to the north.
Changes to the roads surrounding the Clinical Center and changes to the
building itself will remove most of parking lots 20B, 20C, and T4. That
adds up to 400 spaces. Some of those plum parking spots may return when
the CRC is complete. Check back in 2001.
There are alternatives, parking officials note. Parking lots 41A, B, and C and temporary lot T1 at the south end of the campus are rarely full and regular campus shuttle service is provided.
The NIH Office of Research Resources leases 130 parking spaces in Garage
57 in Bethesda and up to 150 NIH cars can park at Mid-Pike Plaza on Rockville
Pike at Montrose Road. You can shuttle to those lots, too. Another 150 spaces
at the Shady Grove Station and 25 at the New Carrollton station are earmarked
for NIHers who can park free and ride Metro.
(by Laura Bradbard)
Dr. Christine Grady, assistant director for clinical science and acting chief of the clinical therapeutics lab, NINR, has been named nurse consultant for the CC Department for Clinical Bioethics. She will also serve as the department's acting chief, according to Dr. John Gallin, CC director.Dr. Ezekiel Emanuel, an oncologist from the Dana-Farber Cancer Institute at Harvard, has been named director of that department and will move into the position full-time in 1997. Dr. Grady, whose doctorate is in philosophy and bioethics, is a clinical scholar at the Center for Clinical Bioethics at Georgetown University Medical Center and co-director of an interdisciplinary bioethics course for medical and nursing students.
Dr. Larry Green, Pharmacy Department deputy chief, has accepted
a position as manager of professional services with the Amgen Corporation
in Thousand Oaks, Calif. He'll be working with their clinical grants team
to develop phase IV clinical research studies in hematology and oncology.
Dr. Green had been at the Clinical Center for six years. Before accepting
his post here, he was at Johns Hopkins Hospital.


During the project, hours for the cafeteria on B1 will be 6 a.m.- 11 p.m. on weekends. It will close at 11 p.m. on weekdays.
Clinical Pathology's annual holiday auction, which is a benefit for the Patient Emergency Fund and FOCC, will be Dec. 6, 10 a.m.-2 p.m., in room 2C310. To donate baked goods, crafts. or household items, call Norma Ruschell or Khanh Nghiem at 496-4473.
"Our Clinical Center co-workers have taken to heart the campaign's slogan, 'Help Hope Take Shape,'" notes Warren Moyer, assistant campaign coordinator.
"Participation has been phenomenal, reflecting that CC staff recognize the need to support the noteworthy charities participating in the CFC."
Comments?
webmaster@cc.nih.gov
National Institutes of Health (NIH)
Last modified 11/21/96

|
|