Clinical Center News
Genomic sequencing identifies unexpected bacterial diversity on human skin
Take a look at your forearm—freckles, hair, maybe a birthmark. Unseen are an average of 44 species of bacteria that populate normal skin and possibly impact the health of one of the body’s first lines of defense against illness and injury.
This bacterial diversity is just one of the findings of an initial study by NIH researchers published May 29 in the journal Science, an effort to explore the skin’s microbiome—all of the DNA, or genomes, of all the microbes that inhabit human skin.
NIH recently launched the Human Microbiome Project, a part of the NIH Roadmap for Medical Research, to discover what microbial communities exist in different parts of the human body and to explore how these communities change with disease. In addition to skin, that project is sampling the digestive tract, nose, mouth, and vagina.
 |
|
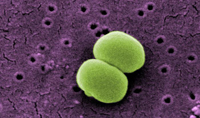
One of the most common bacteria found on human skin is staphylococcus epidermidis, seen above courtesy of the CDC Public Health Image Library. Though usually non-pathogenic, the bacteria can cause an infection in patients with compromised immune systems or indwelling medical devices.
|
Drawing on the power of modern DNA sequencing technology and computational analysis, the research team from the National Human Genome Research Institute, the National Cancer Institute, and the Clinical Center uncovered a far more diverse collection of microbes on human skin than had been detected by traditional methods that involved growing microbial samples in the laboratory.
“The advanced DNA techniques are going to give us the information we need to understand these complex microbial communities, and once we understand their relationships to healthy and diseased skin surfaces, we can identify treatments,” said Dr. Patrick Murray, chief of the CC Department of Laboratory Medicine’s Microbiology Service.
Researchers took skin samples from 20 sites on the bodies of 10 healthy volunteers. “We selected skin sites predisposed to dermatological disorders, sites where microbes have long been thought to play a role in disease activity,” said study coauthor Dr. Maria Turner, senior clinician in NCI’s Dermatology Branch. In the future, their findings will be used to compare the microbial flora of normal skin with that of skin afflicted by diseases such as atopic dermatitis and psoriasis.
Some of each sample went to the genome institute for sequencing and subsequent bacterial identification. The other portion went to the CC Department of Laboratory Medicine for state-of-the-art microbiological techniques to isolate and grow the bacteria sampled from the skin.
NHGRI researchers extracted DNA from each sample and sequenced the 16S ribosomal RNA genes, a gene that is found in all bacteria. The researchers identified more than 112,000 bacterial gene sequences, which they then sorted and classified.
The microbiology laboratory could not isolate most of the bacteria found by the genetic method, Murray said. The new approach found more complex, new, and unexpected bacteria, such as many gram-negative (thin-walled) bacteria previouslythought too fragile to survive on the skin.
“As the genetic approach becomes more user-friendly, it will move into the clinical environment and replace some of our current methods,” Murray said.
The skin sites selected for the Science study represent three microenvironments: oily (beside the nose, upper chest), moist (armpit, behind the knee), and dry (palm of the hand, the buttock). Researchers found that dry and moist skin sites had a broader variety of microbes than did oily skin sites.
The study also shows that, at least among healthy people, the greatest influence on bacterial diversity appears to be body location. For example, the bacteria that live under your arms are more likely to be similar to those under another person’s arm than they are to the bacteria that live on your forearm.
They found considerable variation in the number of bacterial species at different sites, with the most diversity being seen on the forearm (44 species on average) and the least diversity behind the ear (19 species on average).
Another facet of the study intended to identify the least invasive sampling method that would yield an accurate picture of the diversity of skin microbial flora. “Scientists know that the skin is not a completely flat surface. There are hills and valleys caused by skin appendages like hair, oil, and sweat glands,” said Turner. Thus, her team used a swab, a scrape, and a punch biopsy to capture cells on the surface, a couple of layers deeper, and several millimeters into the sweat and oil glands, respectively. “We found we could get as much information as we wanted using the swab, which is totally noninvasive,” she said.
For more information on the Human Microbiome Project, including more findings from this first look at the skin microbial floor, visit the NHGRI Web site at http://genome.gov.
Back to Top
Marburg survivor aids in research
 |
|
Michelle Barnes contracted Marburg hemorrhagic fever in Uganda in December 2007.
|
The first known US case of Marburg hemorrhagic fever—confirmed early this year—ended favorably, as Michelle Barnes of Colorado lives with mild residual effects after surviving the frequently fatal condition.
Barnes traveled to the Clinical Center on June 22 to share her story with NIH staff and offer a blood and DNA sample to the National Institute of Allergy and Infectious Disease’s Vaccine Research Center.
“By studying Michelle’s blood samples, we can study the immune response to natural infection to this rare disease, which could help create a vaccine to prevent the spread of Marburg,” said Vaccine Research Center, Clinical Trials Core, Deputy Chief Dr. Julie (Martin) Ledgerwood. The Vaccine Research Center is evaluating a Marburg DNA vaccine in Phase I and Ib studies—testing the safety and immunogenicity of candidate vaccines.
Marburg hemorrhagic fever is caused by a zoonotic (animal-borne) RNA virus and is in the same family as Ebola. First recognized in 1967 in Marburg and Frankfurt, Germany and in Belgrade, Yugoslavia (now Serbia) in laboratory workers exposed to African green monkeys or their tissues, Marburg boasts a high death rate, up to 88 percent. The fever’s history since has been kept to mostly isolated incidents all in central Africa, with recent outbreaks in the Democratic Republic of the Congo, Angola, and Uganda.
Barnes, 46, contracted Marburg while vacationing in Uganda in December 2007. Three days after her New Year’s Day return from the safari, Barnes started feeling ill to a degree she could not excuse as jet lag or traveler’s diarrhea. She developed symptoms of headache, weakness, fatigue, muscle ache, nausea, vomiting, abdominal discomfort, diarrhea, mental fogginess, and a diffuse rash. Barnes was admitted to Lutheran Medical Center in Wheat Ridge, Colo., with renal failure and spent 12 days in the hospital undergoing diagnostic testing and receiving empiric antibiotics and supportive care. She spent eight days in intensive care, and developed gallbladder inflammation requiring its removal.
Dr. Norman Fujita, Barnes’ infectious disease physician in Colorado, traveled with his patient to NIH to present the case. He recalled considering a number of potential causes for Barnes’ condition based on her travels and exposure —she did, after all, fall in the Nile River while rafting, explore a python cave, and visit the Bwindi Impenetrable Forest to observe the endangered mountain gorillas. Fujita considered various types of viral hepatitis, herpes complex infection, leptospirosis, and “other unusual viral infections” as the cause of her illness. None of his hypotheses checked out.
 |
|
Accompanying Marburg survivor Michelle Barnes (second from right) were her infectious disease doctor from Colorado Dr. Norman Fujita and her sister Dr. Melissa Peters (right). From the VRC, (from left) Dr. Nancy Sullivan, chief of the Biodefense Research Section, and Dr. Julie Ledgerwood, Clinical Trials Core deputy chief, spoke on the research on the disease conducted at NIH.
|
Due to her unique travel and exposure history, on the day after hospital admission, Fujita contacted the Colorado State Health Department which connected him to the Centers for Disease Control and Prevention. Based on the initial information, the CDC offered other diagnostic possibilities including arboviruses, rickettsial infection, and acute schistosomiasis. The center thought the Ebola virus or Marburg fever was possible but unlikely, but still tested for both in Barnes’ arboviral serology—a blood test for insect-transmitted diseases. All tests came back negative.
Barnes was released but still suffered insomnia, fatigue, and mental fogginess that affected her day-to-day activities. In July 2008 she received an e-mail from a journalist friend who had traveled on the safari to Uganda with her that brought to her attention a BBC article on a Netherlands woman who had died of Marburg fever after visiting the same python cave in the Maramagambo Forest in Queen Elizabeth Park. The cave was home to thousands of fruit bats and their guano, thought to be Marburg carriers.
“We were in the same cave, and she had renal failure, too,” Barnes said. “It was too much of a coincidence. I thought, ‘I have Marburg!’”
She urged Fujita to have the CDC do another serology. The repeat blood tests in July were reported by phone to Fujita as negative. Though she hadn’t gotten an official diagnosis, Barnes was convinced she finally knew what had made her ill. With no cure or treatment for Marburg fever, however, she continued on with her life.
In January 2009, Fujita got a call from the CDC that they had confirmed Barnes had Marburg fever, admitting that her earlier tests were borderline, but not conclusive enough to affirm a diagnosis. The positive result ultimately came from reverse transcription polymerase chain reaction and indirect immunofluorescent antibody tests. It was not clear why the tests in January 2008 and July 2008 were borderline by a technique called enzyme-linked immunosorbent assay for the Marburg virus.
Barnes cannot say why she survived and the other visitor to that cave in Uganda did not, but she is glad she is around to help others by allowing researchers to study her samples. She hopes a vaccine will become available to the villages in Africa affected by this deadly disease.
She is not the woman she was two years ago, but Barnes says, “While it is a new normal, I am happy. I lived and am continuing to recover.”
Back to Top
Protocol to reengineer the immune system with survivors' genes renders melanoma patient cancer-free
Mother knows best. Thomas May of Michigan was reminded of that old adage when over lunch his mom urged him to have a suspicious mole checked by his doctor.
A diagnosis of melanoma came in January of 1999, and May, 34 at that time, spent the rest of that year fighting the cancer that spread to his lymph nodes. He enjoyed three years of remission until a December 2003 routine scan showed a mass on his lung. The melanoma was back.
When a regimen (four cycles total) of interleukin-2 therapy failed to eradicate his cancer, May traveled to NIH and was treated in a protocol under Dr. Steven Rosenberg, chief of surgery at the National Cancer Institute, that genetically engineered patients’ own white blood cells to recognize and attack cancer cells.
“I’ve been clean as whistle since August 2006,” May said.
 |
|
Thomas May of Michigan traveled to NIH for treatment and monitoring from 2004 until August 2006 when he was told he was cancer-free.
|
Rosenberg’s study, published in Science in 2006, isolated genes from a cancer survivor that code for the molecules that enable a lymphocyte to recognize cancer and transplanted those genes into the normal cells of current patients. A subsequent study, published in May in Blood, isolated a different receptor more powerful in cancer recognition—growing Rosenberg’s response rate from 12 percent in the first study to 30 percent. May was one of two patients, out of 17, from the first study now disease-free.
Upon the reoccurrence of his melanoma in 2003, May started interleukin-2 treatment at Henry Ford Hospital in Detroit to stimulate the immune system to identify and fight the cancerous cells. After his first two cycles of the interleukin-2, May was suffering from side effects—his skin was bright red and itched constantly—but the tumor had shrunk. Two cycles later, though, an October 2004 scan showed the tumor was growing.
Though he found the news “devastating,” May was not ready to succumb to his cancer. “I couldn’t quit,” he said. “I knew someone, somewhere in these United States had to be working on this thing.”
The doctor who cut out May’s first round of melanoma told him about the NIH and the possibility of a clinical trial exploring new treatments. May called the Clinical Center and sent his medical records for review. Less than a month after he received the bad news from his scan, May came to the NIH for screening for potential protocols.
He was impressed by the gene therapy trial, and was ecstatic to learn a few days after his visit that he’d been accepted. “They make your immune system smarter. That’s just the coolest thing I’ve ever heard,” May said.
Rosenberg’s team infected May’s lymphocytes—a type of white blood cell in the immune system—with the genes to encode specific proteins, T cell receptors, which decorate the outside of the lymphocytes. The T cell receptors act as homing devices in that they recognize and bind to tumor cells, then activate the lymphocytes to destroy the cancer cells.
May made two trips for apheresis—circulating the blood out of the body, separating out one part, and replacing the rest—and received a transfusion of the reengineered cells in mid-January 2005. After two cycles of interleukin-2 and four or five trips between Bethesda and Michigan, May was ready for the scan to report on the treatment’s effect in the early spring of 2005.
“When the doctor has a smile on his face when he comes in the room, you know it’s going to be good news,” May said. His tumor had shrunk by 50 percent. He returned to the CC every three months for a scan and on August 1, 2006, May heard the news he’d longed for—“your scans show no signs of cancer.”
May knows he is one of the blessed ones and credits his faith and strong prayer support for helping him reach his current state. May said he owes a special debt to his mother and father. Though no picnic for him, he feels the ordeal was harder on them as they saw what he went through. “I just couldn’t give up!” May said.
Rosenberg is pleased with his patient’s positive outcome, but quick to point out that his work is far from over. “We’ve been working hard to develop new treatments for patients with cancer and the ability to genetically manipulate the immune system of humans opens new possibilities for treatment. Mr. May received a highly experimental treatment: gene therapy. But there are many improvements that will be necessary before we’ll be satisfied that this is an effective treatment for more patients,” he said.
Back to Top
BTRIS Phase 1 launch set for July 30
User support center will help staff learn to navigate the new informatics system
The Biomedical Translational Research Information System is an NIH-wide research data repository designed to assist researchers with managing and reporting information throughout the research cycle. Following the launch at the end of the month, NIH principal investigators with active clinical protocols will be invited by e-mail to register for access to BTRIS. Designed to extend the capabilities of the NIH Clinical Research Information System that researchers currently use for managing patient care, BTRIS will allow researchers to:
- access data by protocol, across multiple protocols, or by disease state;
- combine patient care data with data generated by research activities;
- and have broad access to de-identified data for such purposes as hypothesis generation or patient recruitment.
 |
|
BTRIS enterprise director Dr. Jim Cimino (left) shows NIAID’s Dr. Richard Davey how to navigate the new system in the BTRIS-User Support Center in the Hatfield Building’s room 4-2480.
|
BTRIS will be deployed in two phases. Phase 1 launches July 30, allowing principal investigators with active protocols to view their patients’ identified data from the Clinical Research Information System, the Clinical Research Information Management System of NIAID, and the NIAAA database. Phase 2 will allow NIH researchers with a user license access to de-identified data from the Clinical Research Information System, the NIAID system, and NIAAA starting on September 15. The repository of de-identified information will include data from the approximate 1,500 active protocols and 6,000 terminated protocols.
When fully deployed, BTRIS will provide access to valuable information and tools such as demographic data, vital signs, laboratory data, medication administration, clinical documentation, and image data from the Clinical Center, as well as similar kinds of data from other institute and center research information systems. The use of these data will be governed by the BTRIS Data Access and Use Policies and the BTRIS Code of Conduct.
Licenses to BTRIS will be provided to principal investigators who are NIH employees and who have completed the BTRIS Data Access Computer Based Training. Principal investigators with active protocols will be contacted in late July or early August regarding access to BTRIS.
A BTRIS-User Support Center in the Hatfield Building’s room 4-2480 will provide walk-in support for users from 8:30 am to 5:00 pm, Monday through Friday from July 30 through October 31.
Dr. Jim Cimino, chief of Laboratory for Informatics Development and director of the BTRIS enterprise will showcase access and query capabilities of the BTRIS data repository at a town hall meeting on Tuesday, September 15 at 2 pm in Lipsett Amphitheater. Archived videocasts of the BTRIS lecture series featuring leading figures in the study and use of translational information systems from academic centers across the United States are available at http://btris.nih.gov.
 |
|
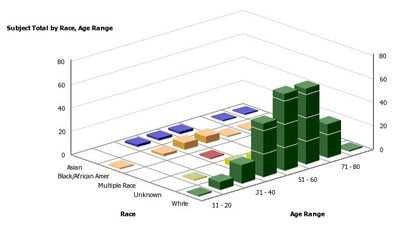
BTRIS will allow researchers to pull patient information from a variety of databases, allowing a comprehensive look for analysis and comparison at study details such as race and age range, as shown in the above graph.
|
Back to Top
Decker Lecture examines graft-vs-tumor effect
 |
|
Dr. Richard Childs of NHLBI was honored with the 2008 Distinguished Clinical Teacher Award in September.
|
It was something of a family affair at the Clinical Center’s Lipsett Amphitheater on June 10 as Dr. Richard Childs, chief of the Transplantation Immunotherapy Section in the Hematology Branch of the National Heart, Lung and Blood Institute, gave the Sixth Annual John Laws Decker Memorial Lecture as recipient of the 2008 Distinguished Clinical Teacher Award.
After being introduced by CC Director Dr. John I. Gallin as “an exceptional mentor and teacher,” Childs smiled as he acknowledged his wife’s presence in the audience. “This is the first time she’s ever heard me give a medical lecture,” he said. “So I’m hoping that after that introduction I won’t have to do the dishes this week.”
Childs was honored with the Distinguished Teacher Award on Sept. 10, 2008. He received his medical degree from Georgetown University before joining NIH for a fellowship in medical oncology at the National Cancer Institute and a hematology fellowship at the NHLBI. He was appointed as a tenure-track investigator in the Hematology Branch of the NHLBI in 1999 and received tenure in 2006. Childs recently attained the rank of Captain in the US Public Health Service.
His research has focused on tumor immunology and allogeneic immunotherapy (patient receives blood-forming stem cells from genetically similar but not identical donor ie. sibling) to treat nonmalignant hematological disorders, hematological malignancies, and solid tumors. Childs was the first to establish the existence of a graft-versus-tumor effect—immune response to a person’s tumor cells by immune cells present in a donor’s transplanted tissue, such as bone marrow or peripheral blood—that could cure patients with metastatic kidney cancer.
 |
Dr. John Laws Decker’s daughter Dr. Megan Molaro (right) and son David Decker (second from left) and his wife Lisa Greenlees (left) joined CC Director Dr. John I. Gallin (middle) and Dr. Richard Childs.
|
As part of the CC’s Grand Rounds Great Teachers series, Childs presented the lecture “Transplanted Allogeneic T-cells Identify a Viral Corpse Resurrected in Renal Cell Carcinoma,” characterizing studies investigating the graft-versus-solid tumor effect in metastatic kidney cancer patients who have undergone hematopoietic (from bone marrow or blood) stem cell transplantation.
Childs said one of the goals of the investigation was to move from “proof to principle” in establishing more effective allogeneic transplant trials. “Clinical observations showed that in the majority of patients that had a disease response, tumors would grow the first couple of months after the procedure, followed by tumor regression,” Childs said.
The annual lecture is presented in memory of former CC Director Dr. John Laws Decker who died in 2000. He served as director of the CC and as NIH associate director for clinical care from 1983 until his retirement in 1990, after which he was named scientist emeritus. His children, Dr. Megan Molaro and David Decker, attended the lecture and a reception afterwards.
A videocast of Childs’ lecture is available in the Grand Rounds archives at http://videocast.nih.gov.
Back to Top
NIH Mind-Body Week cancelled
The NIH Mind-Body Week scheduled for September 8 to 11 has been cancelled. The NIH was given the enormous opportunity and attendant responsibility of funding an unprecedented $8.2 billion to support scientific research priorities as part of the American Recovery and Reinvestment Act of 2009. The period proposed for holding the Mind-Body Week is exactly when much of the NIH will be focused on is ensuring that all of the successful ARRA applicants receive their awards prior to the end of the NIH fiscal year on September 30. The weeks’ planners regret any inconvenience caused by the change in plans.
Back to Top
CC partners with Inn on teen program
The Clinical Center Rehabilitation Medicine Department’s Recreation Therapy Section revamped an old collaboration with The Children’s Inn and will offer a monthly program specifically for teenage patients and teenage siblings of patients.
“The events promote social interaction with peers and allow teens to talk about what they’re facing with others having a similar experience,” said Recreation Therapy Chief Donna Gregory.
The program kicked off on June 9 with tie-dying. Recreation therapist Laura Maring (standing) and The Children’s Inn intern Caitlin Farren (left) helped patient Nathan Fried and patient sibling April Thomas make rainbow T-shirts for themselves and April’s brothers. The Recreation Therapy Section and The Children’s Inn will also hold a Teen Retreat on July 27 and 28 to engage this group in activities such as the Young Adult & Teen Council discussion panel and a collective art project.
Back to Top
Registration for Principles of Clinical Pharmacology open
The Principles of Clinical Pharmacology course, sponsored by the Clinical Center, will begin in Lipsett Amphitheater on September 3. The course will be held Thursdays from 6:30 pm to approximately 7:45 pm through April 22.
“Many medical schools don’t offer formal courses in clinical pharmacology,” said CC Director Dr. John I. Gallin. “This program covers what researchers need to know concerning the clinical pharmacologic aspects of drug development and use.”
The course covers topics such as pharmacokinetics, drug metabolism and transport, assessment of drug effects, drug therapy in special populations, and drug discovery and development. Since first offered 12 years ago, Principles of Clinical Pharmacology has expanded beyond the CC to include a number of off-site partners. Last year approximately 206 students from 13 long-distance partners registered for the course in addition to the approximately 345 enrollees at the NIH.
“We have been very pleased with the great interest generated by this course,” said Dr. Frederick P. Ognibene, deputy director for educational affairs and strategic partnerships at the CC.
The course director of Principles of Clinical Pharmacology is Dr. Juan Lertora, director of CC Clinical Pharmacology and a member of the Office of Clinical Research Training and Medical Education. “We have assembled an outstanding faculty for this course, drawing from the scientific staff at the NIH, the Food and Drug Administration, the pharmaceutical industry, and many prestigious academic institutions in the United States,” Lertora said.
The faculty, led by former course director, Dr. Arthur J. Atkinson, Jr., prepared and edited a textbook, Principles of Clinical Pharmacology, Second Edition (2007), which follows the sequence of the course lectures. This textbook is available in the Foundation for Advanced Education in the Sciences bookstore on the Magnuson Building’s B1 level and at www.amazon.com.
Registration is open and free to all interested individuals not taking the course for graduate credit. Principles of Clinical Pharmacology may be taken for graduate credit through the Foundation for Advanced Education in the Sciences as PHAR 500 I and PHAR 500 II. Contact them directly at 301-496-7976. Certificates of participation will be awarded at the end of the course to all students who attend at least 75 percent of the lectures.
To register and for additional information, visit www.cc.nih.gov/training/training/principles.html. The deadline for registration is August 21, 2009. For further assistance, contact Donna Shields at 301-435-6618.
Back to Top
CC celebrates Sickle Cell Awareness Day
 The Pulmonary and Vascular Medicine Branch of the National Heart, Lung, and Blood Institute celebrated the First Annual Sickle Cell Disease World Day on June 19.
The Pulmonary and Vascular Medicine Branch of the National Heart, Lung, and Blood Institute celebrated the First Annual Sickle Cell Disease World Day on June 19.
The day is the result of the United Nations resolution that called for the recognition of sickle cell disease as a public health problem and “one of the world’s foremost genetic diseases.”
Current estimates suggest that over 250 million people worldwide are affected by this heritable blood disorder. The group noted the day with a public discussion between researchers and patients followed by a reception in the Clinical Center.
In attendance were Dr. Stewart Levine (middle row, right), acting chief of the Pulmonary and Vascular Medicine Branch; Dr. Caterina Minniti (front row, middle), the branch’s director of clinical services; Beatrice Bowie (front row, third from left), patient advocate; Ihsan Rogers (back row, right), social worker; and Dr. Willy Flegel (middle row, second from left), chief of the CC’s Department of Transfusion Medicine’s Laboratory Services Section
Back to Top
Long-time Clinical Center chaplain dies
The Rev. Eugene Linehan, a former chaplain in the Clinical Center’s Spiritual Ministry Department, died May 23 at age 87.
Linehan served the CC for 27 years, joining the staff in 1972 and retiring in 1999. According to an obituary in The Washington Post, Linehan remained in the area after leaving NIH as a pastoral minister at Georgetown Prepatory School until this year. Linehan had recently moved to Manresa Hall, a Jesuit community in Merion Station, Pa.
He entered the Jesuit seminary at age 18 and trained for 15 years. During that time, Linehan earned a philosophy degree from the old Woodstock College and a master’s degree in theology from what is now the Weston Jesuit School of Theology in Massachusetts, reported The Washington Post.
Over his varied career, Linehan taught high school English and led a Jesuit retreat center.
Upon his retirement from the CC, Linehan spoke on the power of daily mass and prayer for patients and his admiration of the pediatric patients. He told the CC News in 1999 of one young patient who told the reverend about his impending death, “I have lost the battle, but I’ve won the war.”
“These children and patients here who can accept illness and death with complete serenity are a blessing,” Linehan said.
A memorial mass was offered for Linehan in the CC Inter-faith Chapel on June 15.
Karen Baker, a nurse practitioner in the CC’s Pain and Palliative Care Service, remembers Linehan’s “bright effect and availability” to patients, families, and staff. “It didn’t matter what religious affiliation someone was, he was very comfortable in his supportive role,” Baker said. “He truly was a caregiver.”
Back to Top
Staffers join Team NIH to race for a cure
|
More than 40 staffers turned out on a sunny Saturday morning for the 2009 Susan G. Komen Global Race for the Cure on June 6. Team NIH joined a crowd of nearly 45,000 people who ran or walked the 5 kilometers (3.1 miles) to support the fight against breast cancer.
Susan G. Komen for the Cure was founded in 1982 by Nancy G. Brinker after she promised her dying sister she would do everything in her power to end breast cancer.
Today, Komen for the Cure is the world’s largest grassroots network of breast cancer survivors and activists fighting to save lives, empower people, ensure quality care for all, and energize science to find the cures. With more than $1.3 billion invested to date, Susan G. Komen for the Cure is the world’s single largest source of nonprofit funds dedicated to curing breast cancer—second only to the US government.
Team NIH plans to participate in the 2010 Komen Global Race taking place on June 5, 2010. Register today at http://globalraceforthecure.org/. Click Join a Team, then enter Team NIH and choose US government agency from the dropdown.
|
 |
Back to Top
News Briefs
Weekend access for patients
As of July 3, all Clinical Center patients and visitors traveling to the NIH on weekends and holidays should enter campus via the Commercial Vehicle Inspection Facility. The entrance into the facility can be accessed from the south-bound lanes of Rockville Pike (Route 355) between North Drive and Wilson Drive.
The Gateway Center Vehicle Inspection will continue normal operations weekdays from 5am to 10pm. Pedestrians should continue to enter campus using the NIH Gateway Center 24 hours a day, seven days a week. A special entrance for patients and patient visitors at the intersection of West Cedar Lane and West Drive, directly north of the main entrance of the Hatfield Building, is open Monday through Friday from 7 am to 7 pm.
Visit www.cc.nih.gov/about/visitor/dir_to.shtml for directions to the CC. For questions about campus access, contact the Office of Research Services at 301-594-6677. Tune the radio to 1660 AM for further NIH traffic and parking advisories.
CC Piano Concert Series: upcoming performance
Ervand Kristosturyan and Tigran Hovhannisyan will perform works by Chopin, Rachmaninov, Khachaturian, Piazzolla, and others on July 31 on the Steinway grand piano in the Clinical Center atrium at 12 pm.
Kristosturyan has been playing piano since the age of 6. He attended Sayat-Nova School of Music in Yerevan, Armenia, then took private lessons after moving to the United States. He did his undergraduate work at The George Washington University, and is currently an NIH fellow applying toward his medical degree. In his spare time, Kristosturyan accompanies classes at The Washington Ballet and directs the NAREK Bell Choir at St. Mary’s Armenian Church.
Hovhannisyan started playing piano early and graduated from Sayat-Nova, majoring in forte-piano, at age 14. His fondness for classical saxophone came when he attended Yerevan State Conservatory, Concervatoire de Music de Geneve, and Concervatoire de Lausanne. He is a winner of the French-Armenian International Music Competition and the British-Armenian Music Festival on saxophone. Living in Washington DC, Hovhannisyan teaches private piano and saxophone lessons.
Follow the CC on Twitter
Tweet, tweet! The Clinical Center has joined the social media network Twitter. As Wikipedia describes it, “Twitter is a free social networking and micro-blogging service that enables its users to send and read each others’ updates, known as tweets. Tweets are text-based posts of up to 140 characters, displayed on the author’s profile page and delivered to other users—known as followers—who have subscribed to them.”
The CC is listed under NIHClinicalCntr at http://twitter.com/NIHClinicalCntr and links to the latest podcasts and CC News online, announces lectures and events, and gives information for current and potential patients. Sign up for your own free account and follow us.
If you would like to submit any content to be posted to the NIHClinicalCntr page, contact Maggie McGuire at 301-594-5789.
Back to Top
New clinical research protocols
The following new clinical research protocols were approved in May:
Changes in the Posterior Parietal Cortex—Primary Motor Cortex Pathway Induced by Motor Training, 09-N-0146, Mark Hallett, MD, NINDS
Establishing Fibroblast-derived Cell Lines from Skin Biopsies of Patients with Immunodeficiency or Immunodysregulation Disorders, 09-I-0133, Helen C. Su, MD, NIAID
Host Factors in Invasive and Recurrent Staphylococcus Aureus Infection, 09-I-0157, Steven M. Holland, MD, NIAID
A Pilot Study of a Thrombopoietin-Receptor Agonist (TPO-R Agonist), Eltrombopag, in Aplastic Anemia Patients with Immunosuppressive-Therapy Refractory Thrombocytopenia, 09-H-0154, Yong Tang, MD, NHLBI
The Effect of NK1R Antagonism on Alcohol Craving and PTSD Symptoms in Alcohol Dependent Patients with PTSD, 09-AA-0136, David T. George, MD, NIAAA
Natural History of Autoimmune Diabetes and Its Complications, 09-DK-0140, David M. Harlan, MD, NIDDK
Back to Top
This page last updated on 12/14/2017


 The information on this page is archived and provided for reference purposes only.
The information on this page is archived and provided for reference purposes only.