First-in-human gene therapy launches with pediatric patient with rare disease
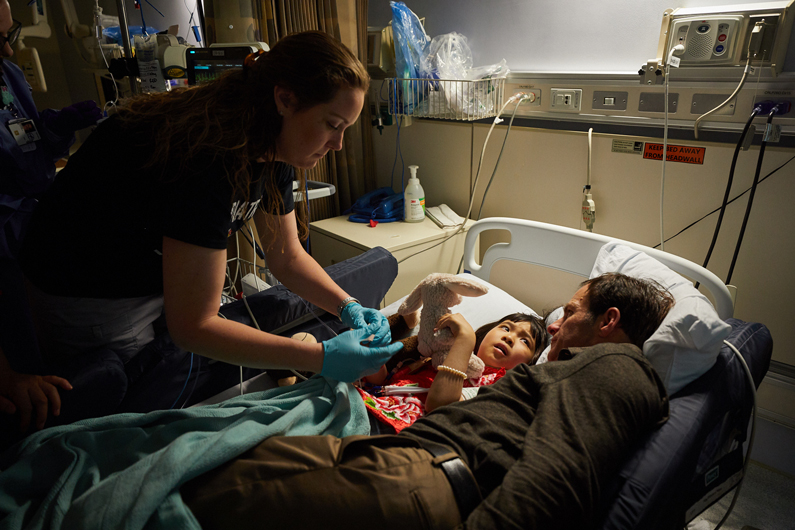


In May, a ten-year-old girl named JoJo received a gene therapy for an extremely rare neurogenerative disease called GM1 gangliosidosis.
GM1 progressively destroys nerve cells (neurons) in the brain and spinal cord, leading to muscle and movement weakness, difficulty swallowing and a shortened life expectancy. The first-in-human gene therapy trial was led by Dr. Cynthia Tifft with the National Human Genome Research Institute and partners at the University of Massachusetts and Auburn University. The therapy provides a replacement, or normal, copy of the gene GLB1. The gene is encapsulated in a virus that has shown it targets the central nervous system.
"It's like a viral infection. The viral code dumps the DNA into a cell," Tifft said. "All the guts of the virus (the DNA of the virus) has pretty much been eliminated to make room for the gene you want. The gene we want is sitting in the cell and will begin to make the enzyme that is deficient in the child."
JoJo is one of 35 patients who are a part of an NHGRI natural history study for GM1.
"There was very little known about the disease until about 10 years ago when we started doing a natural history study," said Tifft, principal investigator of the trial. "Based on data that we obtained doing our natural history study in the Clinical Center, the only place we could have ever done it, we basically had enough data to launch a gene therapy trial."
As for most scientific research, Tifft said this has been quite the odyssey. JoJo has been coming here for four years. She used to talk and walk quite well, but now only has the ability to say a few single words and cannot hold herself upright.
When it came time for the 30 minute IV infusion of the gene therapy, a medical advancement a decade in the making, Tifft recalls the moment feeling "almost spiritual."
"It was kind of surreal almost because you think you know what's going to happen and how things are going to work," Tifft said. "While JoJo slept peacefully, 30 of us stood quietly in her dimly lit room listening to the infusion pump and watching her sleep."
While it's still early to tell, at about three weeks post-gene transplant, Tifft said "JoJo stood independently for five minutes. I haven't seen her stand like that for almost two years. That makes me really optimistic."
The care team plans to bring in the second patient mid-August and the third patient mid-November. They will follow each patient for five years, post-gene therapy.
- Molly Freimuth