First in Human: Discovery Documentary on the NIH Clinical Center
Originally Aired
AUGUST 10, 2017 - PART I
AUGUST 17, 2017 - PART II
AUGUST 24, 2017 - PART III
NIH Clinical Center Media Contact
301-594-5789
ccpressgroup@cc.nih.gov
First In Human
First in Human is a documentary capturing the real-life experiences of doctors, researchers, staff, patients and their caregivers, at the National Institutes of Health Clinical Center. For over a year, Discovery, in collaboration with John Hoffman ("Weight of the Nation," "Sleepless in America"), was embedded in the Clinical Center to capture the challenges faced in diagnosing and treating diseases. Narrated by Jim Parsons ("The Big Bang Theory," "Hidden Figures"), the three-episode series showcases the innovative work that takes place within NIH’s Building 10 and provides an in-depth look at the reality of experimental medicine in clinical trials.
View the NIH News Release. View the NIH Director's Blog: Aug. 3, 2017 and Aug. 10, 2017.
Episodes are available through Discovery Go and on demand. Any viewer within the United States with a cable login can watch episodes on the Discovery webpage. View more ways to watch the series.
Take Action: Volunteer for a Clinical Trial
To learn more about the clinical trials featured in the series, or how to participate in a trial, see below.
All of the patients who come to the NIH Clinical Center participate in a clinical trial. Clinical trials look at new ways to prevent, detect, or treat disease. Treatments might be new drugs or new combinations of drugs, new surgical procedures or devices, or new ways to use existing treatments. The goal of clinical trials is to determine if a new test or treatment works and is safe.
Patient Recruitment at the NIH Clinical Center
Call toll free: 1-866-999-5553 (TTY 1-866-411-1010)
Se habla espanol. Email prpl@mail.cc.nih.gov
Visit ClinicalTrials.gov
Clinical Trial: Anti-CD22 Chimeric Receptor T Cells in Pediatric and Young Adults With Recurrent or Refractory CD22-expressing B Cell Malignancies
This study investigates whether giving anti-CD22 Chimeric Antigen Receptor (CAR) cells to people with certain cancers is safe and effective. It is designed for people ages 1-30 with a leukemia or lymphoma that has not been cured by standard therapy. This therapy takes blood cells from a person, changes the cells in a lab and then gives them back to the person. Researchers use an anti-CD22 gene, a virus and an immune receptor to change the cells.
View trials at the NIH Clinical Center on Leukemia and Lymphoma.
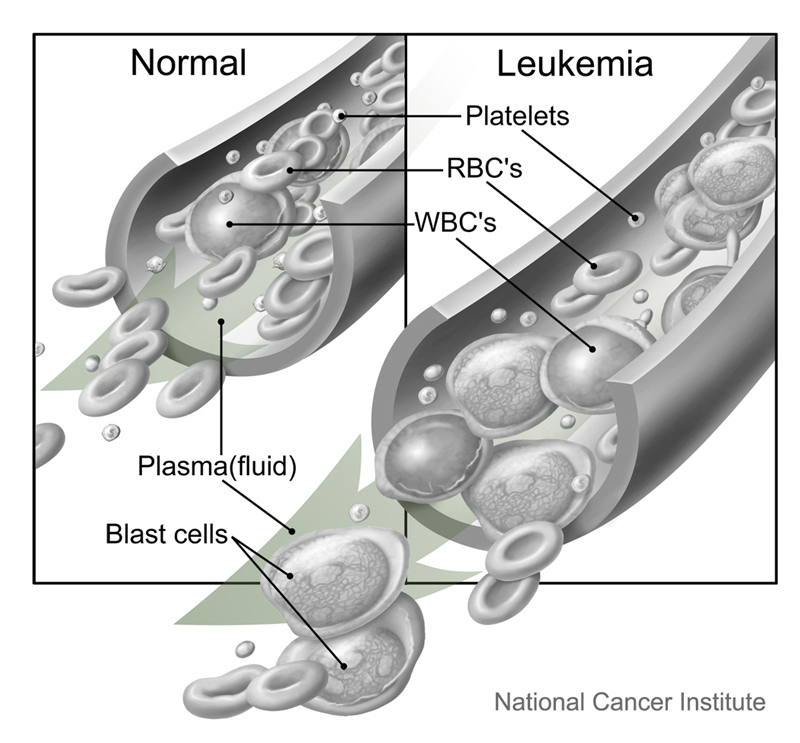
This study investigates whether adding pembrolizumab to cell therapy is safe and effective to shrink melanoma tumors. It is designed for people ages 18-70 years with metastatic melanoma. This is an experimental cancer therapy that takes young tumor infiltrating lymphocytes (Young TIL) cells from a person's tumors and grows them in a lab. The cells are then returned to the person. Researchers think adding the drug pembrolizumab might make the therapy more effective.
View trials at the NIH Clinical Center on Melanoma.
People with anemias often have damage to other organs such as the kidneys, which can be further damaged by the chemotherapy. Only approximately 20 percent of patients have a full-matched donor, making treatment for many people with anemias unavailable. However, 90 percent of patients may have a half-matched donor, but using a half-matched donor increases the toxicity of bone marrow transplantation. This protocol will study if half-matched donor cells, low-intensity radiation, immunosuppressant drugs, and no chemotherapy will be effective in treating patients with sickle cell disease and Beta-thalassemia. The study will also determine the effectiveness of cyclophosphamide, an immunosuppressant drug, in preventing rejection of the donor cells.
View trials at the NIH Clinical Center on Sickle Cell Disease.

Clinical Trial: Study of Clinical Features and Genetics of Hyperimmunoglobulin E Recurrent Infection
In this observational study, the pathogenesis of this disease is being investigated. We seek to enroll patients and families with a confirmed or suspected diagnosis of Hyper IgE Syndromes (HIES) for phenotypic and genotypic study as well as disease management. These individuals suffer from extensive viral infections and have a high incidence of malignancy/mortality. Patients will be examined by a multidisciplinary team and followed longitudinally. We hope to better characterize the clinical presentation of HIES and to identify further genetic etiologies.
View trials at the NIH Clinical Center on Job's Syndrome.
Learn More: NIH Resources
FIRST IN HUMAN is produced by McGee Media for Discovery Channel. The series is directed by John Hoffman; produced by, John Hoffman, Beth Wichterich, and Michael Epstein; narrated by, Jim Parsons; executive producers Dyllan McGee, Jim Parsons, Todd Spiewak, and Eric Norsoph; producer, Jon Bardin; supervising producer, Stacia Thompson; senior editor, Adriana Pacheco; director of photography, Simon Schneider. For Discovery Channel, supervising producer, Jon Bardin; executive producer, John Hoffman.